by Kenny Rogers
Blog was originally posted by me April 2019
Love My Vaporizers, Pipes, E-liquids, Products and Marijuana
We will discuss benefits of Blueberry (fem) near the end of this blog.
Radiation therapy can take place on its own, but it's frequently combined with chemotherapy as a comprehensive cancer treatment program. Radiation therapy differs from chemotherapy — it is used to treat just the tumor, so it affects only the part of the body that has cancer.
Chemotherapy and Radiation Therapy? What Are the Differences?
What Is Chemotherapy?
Chemotherapy, or chemo, is a process in which drugs are used to treat cancer.
It is a “systemic” treatment — working through the whole body to prevent the spread of the disease. The drug(s) used will vary depending on the type and stage of cancer as well as the patient’s age and health. The goal of chemotherapy is to stop the spread of cancer to other parts of the body.Chemotherapy is administered by a medical oncology (cancer) health professional, typically a nurse or doctor. Chemo can be delivered as an outpatient procedure, in a hospital, a doctor’s office, or even at home in any of the following ways:
- Injection into muscle, vein, or artery
- Orally
- Injection into the body (such as the abdomen)
- Direct skin application
Chemotherapy side effects
Chemo side effects vary depending on the type and amount of chemotherapy drug used and how the body reacts to it. Because chemotherapy drugs travel through the body, they can also impact healthy cells, leading to a variety of side effects.
Chemo is designed to kill fast-growing cancer cells, but this can sometimes lead to side effects involving the body’s other, healthy fast-growing cells.
- Blood forming cells in the bone marrow (anemia, increased risk of infection, bruising)
- Hair follicles (temporary hair loss)
- Cells in the mouth, digestive and reproductive tract (nausea, loss appetite, constipation, diarrhea)
Some chemo drugs can damage cells in the heart, kidneys, bladder, lungs, and nervous system. Your doctor monitors you closely and may prescribe medicines to protect your body’s normal cells. There are also medicines to help relieve side effects.
What Is Radiation Therapy?
Radiation therapy is the use of high-energy particles or waves to destroy or damage cancer cells.
Radiation is delivered using special equipment that sends high doses of radiation to the cancer cells or tumor. Radiation can also affect healthy cells, however, normal cells can repair themselves, while cancer cells cannot.
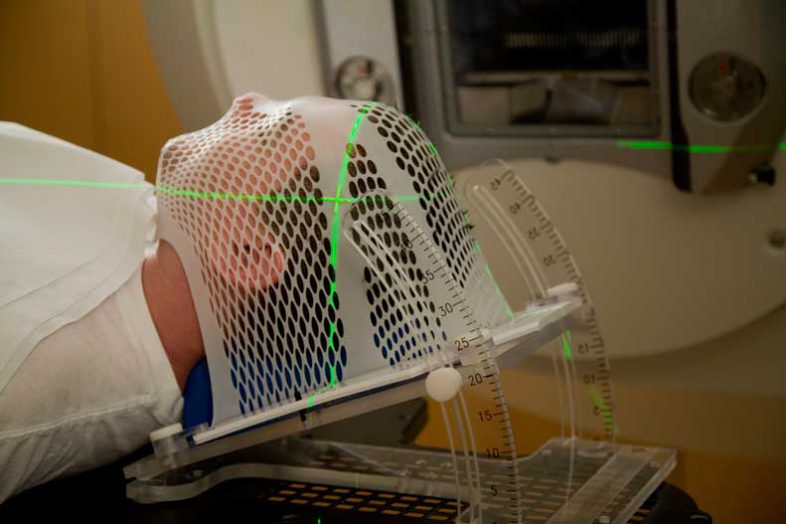
Sometimes radiation is used to treat cancer, or it may be used to help you feel better, such as to minimize bone pain, for example. Radiation therapy can take place on its own, but it’s frequently combined with chemotherapy as a comprehensive cancer treatment program.
Radiation therapy differs from chemotherapy — it is used to treat just the tumor, so it affects only the part of the body that has cancer.
Types of radiation therapy
Radiation can be administered in two ways: internally or externally:
External: External beam radiation is delivered from a machine. It is very similar to receiving a chest X-ray. Most people are treated five days a week for one to 10 weeks, depending on the type and location of cancer, their overall health, and other factors. The treatment only takes a few minutes, and is not generally given over the weekend.
You will be asked to lie flat on a treatment table, under the radiation machine. Other parts of your body may be protected with special shields or blocks to prevent the radiation from going to those areas.
External treatments include:
- 3D conformal radiation therapy after the tumor is mapped through imaging, beams of radiation treat the cancerous tumor.
- Intensity-modulated radiation therapy (IMRT) gives the radiation oncologists the ability to more precisely “custom sculpt” the shape of the tumor. This helps deliver the right amount of radiation more accurately, as well as helps to preserve healthy tissue surrounding the tumor.
Internal: Radiation that is placed inside of the body is called internal radiation therapy or brachytherapy. A radioactive source, called an implant, is placed directly to the tumor or near the tumor. This delivers large doses of radiation to directly to the source of your cancer. These implants may look like a wire, pellet, or seeds.
If the implants are left in your body, you may be given special instructions such as to limit your time with and/or avoid children or pregnant women. After a few weeks to a few months, the implants stop giving off radiation, and you can return to normal activities. The implant, however, will remain in your body forever.
Some implants may be removed after a period of hours or days. Most often, they are administered in a hospital private room, and visitors will only be allowed to stay with you for short periods of time.
The most common types of cancers treated with internal radiation therapy are:
What Cancer Treatment Is Best for Me?
Your treatment plan may be chemotherapy, radiation therapy, surgery, or any combination of these.
It is important that you understand your options, so patients are encouraged to ask questions. If you choose to research the types of cancer treatments available, make sure you’re using a reliable source. Your oncologist and health care team will work closely with you to determine your best treatment plan.
Please allow me the opportunity to share my personal experience with my father's Radiation and Chemo Treatments........
Aug 1998 my father was being treated at the VA center at Ft Hamilton, NY. The pain and suffering that he went thru everyday....He was coming home in excruciating pain. He was curled up and crying like a baby. He was drinking, drinking, drinking to ease the pain. Everyday after treatments, my father was drinking more and more. Beating up and abusing my mother. One day I took the baseball bat and cracked him over his back. The abuse towards my mom stopped! He dranked, dranked, and dranked to eased his pain. He was classified as Stage 4 Cancer on his Liver (no cure)........The VA Center told me to take him home and allow him to enjoy the holidays (which will be his last). Jan 1999 my father died. Radiation and Chemo Treatments did not save his life.
Today, Blueberry is one of the most purchased strains of cannabis on the medical marijuana market. It’s the recipient of the best indica of the year award from the High Times Cannabis Cup. The blueberry flavor makes it pleasing to the tongue, and the high lasts for quite a while. This strain grows best outdoors as it needs a lot of open space to grow wide and bushy.
Medical Uses For Blueberry Strain
Blueberry is infamous for its relaxing abilities. That’s why it’s so highly recommended by both physicians and medical marijuana dispensaries. This indica powerhouse is an effective stress management tool because it restores calmness and promotes relaxation.
Blueberry is a very beneficial strain for medical cannabis patients with chronic ailments. CBD at this level helps to treat some of the most aggressive pain symptoms and easily treats insomnia and depression.
Pain Management
There are other medicinal uses for Blueberry as a pain management tool. It has also been known to relieve uncomfortable symptoms related to the following:
- Headaches
- Migraines
- Cramps
- Menstrual cramps
- Muscle spasms
Mind Management
Blueberry not only relaxes the mind, but it also delivers overall body relaxation as well. So, it’s able to provide medicinal advantages to those suffering from other debilitating symptoms that affect patients on a non-physical level. Some include:
- Cancer Patients – Helps with loss of appetite and extreme nausea caused by chemotherapy
- Loss of Appetite – Increases appetites, beneficial for those suffering from anorexia, HIV, etc...
- Insomnia – Combats restlessness, promotes relaxation, causes sleepiness
- Anti-Psychotic Properties – CBD reduces anxiety which helps with panic attacks and nervousness
- Depression – Delivers THC levels which release happy feelings and helps to relieve stress
Blueberry’s CBD Benefits
Blueberry's CBD levels could help with a number of existing conditions, as well as prevention, such as:
- Alcoholism
- Some forms of cancer
- Rheumatoid arthritis
- Huntington’s disease
- Preventing or reduce diabetes
- Nerve/brain damage caused by stroke
- Mad Cow disease and other prion infections
Please share your experience with your family bout with
Chemotherapy and Radiation Therapy!!!!
Tell me and my friends on this site........We feel your pain and sorry for your lost! Your story will be published without any changes.......
Thank you very much for sharing with us.
Cordially.
Kenny Rogers
Publisher










-1-News.jpg)








